Medtronic manufactures and markets a wide range of medical devices, from electronic
pacemakers to catheters and syringes, to provide life-long solutions for people
with chronic disease.
The Need
Medtronic needed a repeatable, efficient way to track part inventories and product
configurations within their manufacturing environment, and their "new MRP system"
lacked the necessary functionality. The manufacturing process required stringent
controls consistently applied and accurately tracked. Operations was increasingly
bogged down with paper forms that were inconsistent, and provided no effective way
to track part relationships or business constraints. Technicians were required to
"sign off" after performing each step in the process for creating each lot in the
manufacturing process. In short, more work was being required of Medtronic personnel
that was producing fewer benefits to the business.
The Solution
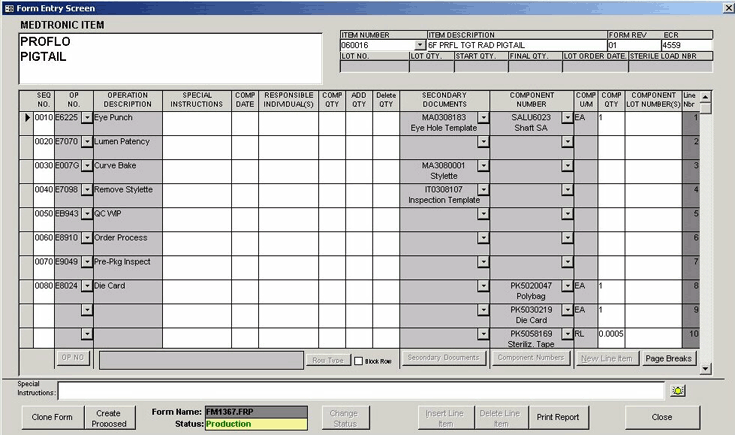
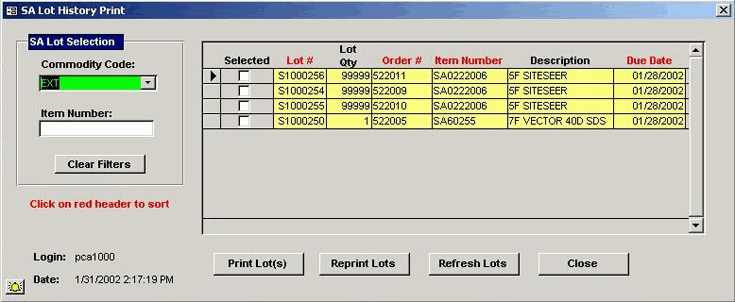
PCA designed and developed a custom parts management and tracking system for Medtronic's
catheter production facilities. The solution supported their existing document-based
parts tracking process and was fully integrated into Medtronic's new MAX MRP system,
existing labeling and barcoding system, and other corporate reporting systems.
Results and Benefits
- Greatly improved efficiency (Some operations realized a 10-fold improvement)
- Reduced human error: significantly improved consistency of product data
- Integration with MRP systems, labeling and barcodes systems
- High quality, highly customized reports
- High system reliability (better than 99.9% uptime)
- Reasonable cost (low cost/benefit ratio)